Navigation
Schistosomiasis Historical perspective: Revisiting the St. Lucia Project a multi-year comparison trial of schistosomiasis control strategies
- Anthony Solomon
- Birgitte Jyding Vennervald
- Charles H. King
- chemotherapy
- Daniel G. Colley
- David Mabey
- Emma Harding-Esch
- hygiene
- Janine Selendy
- Jens Aagaard-Hansen
- Joseph A. Cook
- Julianne A. Ivy
- Pascal Magnussen
- Paul Farmer
- provision of clean water
- Rockefeller Foundation
- sanitation
- Schistosomiasis control strategies
- Schistosomiasis transmission
- Silvio P. Mariotti
- snail control
- st. lucia
- WASH
- water
- World Heath Organization
- Global Health
- Public Health
- Sanitation
- Water
- Water and Sanitation
In 1965, the government of St. Lucia and the Rockefeller Foundation undertook what became a sixteen-year project to determine the optimal strategy for controlling locally-endemic schistosomiasis mansoni. Many of the world’s leading researchers on schistosomiasis control participated in the project, including experts in epidemiology, snail ecology, water and sanitation, social mobilization, clinical trials, immunology, and health economics. In the process, they brought infection levels in the new island nation to an impressive and steady low. Now fifty years later, the island has maintained its control of the parasite and may be on the cusp of achieving national Schistosoma mansoni elimination status.
 The town of Soufrière, St Lucia,: as seen from the road approaching it from the north, and in the background the twin peaks of Petit Piton and Gros Piton. By Gzdavidwong at Chinese Wikipedia - Transferred from zh.wikipedia to Commons., CC BY-SA 3.0, https://commons.wikimedia.org/w/index.php?curid=2326022
The town of Soufrière, St Lucia,: as seen from the road approaching it from the north, and in the background the twin peaks of Petit Piton and Gros Piton. By Gzdavidwong at Chinese Wikipedia - Transferred from zh.wikipedia to Commons., CC BY-SA 3.0, https://commons.wikimedia.org/w/index.php?curid=2326022
In their chapters on “Schistosomiasis” in the 2 books, Water and Sanitation-Related Diseases and the Changing Environment: Challenges, Interventions, and Preventive Measures (the 1st volume without the word “Changing”), co-authors Pascal Magnussen1, Birgitte Jyding Vennervald2, and Jens Aagaard-Hansen3 write in their Introduction (an excerpt):
“Schistosomiasis, a fresh water-related, helminth infection, is considered the second most important parasitic infection after malaria in terms of public health impact 1. It is endemic in almost 80 countries in the Americas, Africa and Asia, with the highest burden of infection and disease on the African continent, especially in sub-Saharan countries. 600-700 million people are estimated to be at risk of infection and approximately 160-200 million individuals to be infected.”
1. King CH, Dangerfield-Cha M. The unacknowledged impact of chronic schistosomiasis. Chronic Illn. 2008;4:65-79.
The following is an Open-Access Article from PLOS Neglected Tropical Diseases:
Historical perspective: Revisiting the St. Lucia Project, a multi-year comparison trial of schistosomiasis control strategies
By Julianne A. Ivy1, Charles H. King1,2*, Joseph A. Cook3, Daniel G. Colley2
1 Center for Global Health and Diseases, School of Medicine, Case Western Reserve University, Cleveland, Ohio, United States of America, 2 Schistosomiasis Consortium for Operational Research and Evaluation, University of Georgia, Athens, Georgia, United States of America, 3 Gillings School for Global Public Health, University of North Carolina, Chapel Hill, North Carolina, United States of America
Introduction
In 1965, the government of St. Lucia and the Rockefeller Foundation undertook what became a sixteen-year project to determine the optimal strategy for controlling locally-endemic schistosomiasis mansoni. Many of the world’s leading researchers on schistosomiasis control participated in the project, including experts in epidemiology, snail ecology, water and sanitation, social mobilization, clinical trials, immunology, and health economics. In the process, they brought infection levels in the new island nation to an impressive and steady low. Now fifty years later, the island has maintained its control of the parasite and may be on the cusp of achieving national Schistosoma mansoni elimination status. There are many other countries still fighting endemic schistosomiasis, and for them, achieving elimination might seem an elusive goal. However, the research evidence from the St. Lucia project, as documented in its nearly 140 research publications and as summarized in book form by Peter Jordan in 1985 [1], provides many lessons that can be applied to countries battling Schistosoma transmission today (Box 1). For readers interested in the details of study design and the full results of the trials, we have included supplemental S1 and S2 Files at the end of this review to provide search- able listings of the many scientific reports published by the Project.
The 1965–1981 St. Lucia study was the first large-scale concurrent comparison trial of strategies for control of S. mansoni
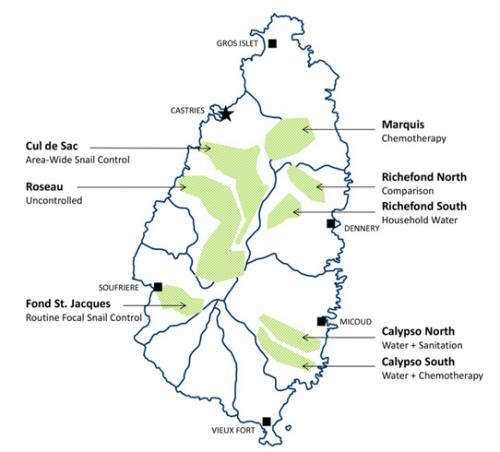
In 1965, the St. Lucia government and the Rockefeller Foundation founded the “Research and Control Department of the Ministry of Health” as a focus for their collaboration on schistosomiasis mansoni control. Their first objective was to get the island’s increasing levels of infection under control. Pre-intervention studies in the high-transmission areas of each targeted valley found a median S. mansoni prevalence of 45% in children [1, p.270], with age-group prevalences reaching up to 91% in individual areas [1, p.53]. Serious illness from schistosomiasis was not uncommon, with a number of children under age 14 having egg counts over 1000, and many more with enlarged livers or spleens [2]. The researchers recognized that while a number of schistosomiasis control methods had been individually tested in trials previously performed in other countries, none had performed head-to-head trials to determine which single method was likely to be most successful in controlling disease or in preventing transmission [1, p.6]. The mountainous terrain of St. Lucia could allow researchers to test individual

control methods in relatively isolated valleys (Fig 1), so the newly-constituted Department designed a large-scale, concurrent comparison study of drug-treatment vs. intermediate-host snail control vs. improvement of water supplies, which was the first study of its kind.
The three main control methods tested in St. Lucia were: (i) targeted chemotherapy, (ii) chemical and environmental snail control (both area-wide and then focal), and (iii) installation of household water supplies with eventual provision of community showers, laundry units, and recreational water facilities. Each strategy presented its own specific set of challenges and technical demands, as noted in Table 1. Installation and maintenance of water supplies proved to be generally the most demanding intervention in terms of supervision, and chemotherapy the least. Snail control required the least amount of direct participation from the community.
Comparing targeted chemotherapy to long-term host snail control and to provision of household water supplies
Drug treatment. Targeted chemotherapy trials were organized in St. Lucia’s Marquis Val- ley in the northeastern sector of the island (Fig 1). Annually from 1973–1976, all children and adults who tested positive for S. mansoni (by the sedimentation stool exam technique) were given treatment with intramuscular hycanthone (1973 and 1974) or oral oxamniquine (1975 and 1976) [3]. This chemotherapy intervention, given alone, resulted in the largest reductions in local prevalence and yearly incidence, as compared to locations having snail control alone or piped water installation alone. Specifically, incidence of S. mansoni in high transmission areas was reduced from 22% to 4% per annum among young children under the age of 10, and existing prevalence was reduced from 41% to 4% [1, p.270]. Drug treatment was the fastest acting intervention, with the most rewarding short-term results [4]. However, a mild increase in S. mansoni prevalence occurred between the third and fourth treatments, and this foreshadowed a later resurgence of transmission in some sectors of the valley [1, p.103].
Water and sanitation. In the southwest part of Richefond Valley, piped water was installed and supplied to 386 individual household taps, serving around 2,000 people; then laundry units, showers, and recreational pools were subsequently provided for the community [5]. A health education program was also implemented in this valley when it became clear that not everyone reduced their contact with streams and rivers after provision of the new water supplies [1, p.212].
 Map
Map
Fig 1. Sketch map of St. Lucia Island, indicating valleys selected for intervention assignments in the 1965–1981 comparison trial of S. mansoni control strategies. Shaded areas indicate the separate valley locations where the different study interventions were tested during the course of the St. Lucia Project. Labels indicate the names of the selected valleys and which interventions were performed at each location. Map created with QGIS v.2.18.10 software (http://www.qgis.org) using a country base map provided by the GADM database of Global Administrative Areas (http://www. gadm.org).
https://doi.org/10.1371/journal.pntd.0006223.g001
Compared to snail control and to chemotherapy, provision of clean water (alone) had the greatest effect on the mean egg output of infected children, yielding a 50% overall reduction in their infection intensity [1, p.270]. Incidence per annum of new S. mansoni infection also decreased from 23% to 13% among children under 10, and overall preva- lence decreased from 56% to 38% [1, p.270]. An additional trial involving installation of water- seal latrines was undertaken in the northern part of Calypso, but due in part to irregular water supplies during a period of drought, this additional intervention did not end up significantly affecting Schistosoma transmission [1, p.255].
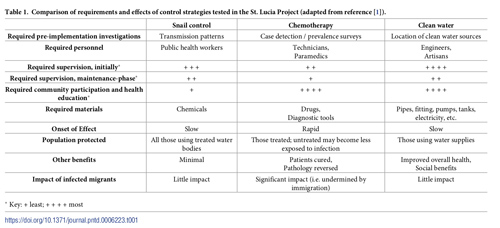 Table 1
Table 1
Snail Control. The primary snail control trials took place in Cul de Sac Valley. After an initial period of three years to establish baseline data and to map breeding sites, the Department implemented area-wide mollusciciding with niclosamide from 1970 to 1975 [1, pp.118/ 131]. This involved initially treating all known snail habitats, and then routinely re-surveying these habitats and treating only those where snails were found. This was performed at 2-week intervals for streams and marshes and at 3 to 4-week intervals for banana drains [1, pp.136/ 165]. This aggressive, wide-coverage campaign brought human incidence per annum of S. mansoni down from 23% to 6% and prevalence from 45% to 24% among children in high- transmission areas of the Cul de Sac Valley [1, p.270]
Even with this success, the investigators realized that the costs and time demands of such intensive intervention would be prohibitively high for many resource-poor countries where schistosomiasis is endemic. As a result, they chose to test a new, less costly alternative, which involved using only focal snail control at specific transmission sites [6]. For this trial, they chose the Fond St. Jacques region of St. Lucia. In this second test, the researchers identified 12 sections of stream where infected Biomphalaria. glabrata had been found and where humans had frequent contact with the water [1, p.181]. These were selected as the most likely sites of transmission and thus were made the sole locations for monthly niclosamide treatments between 1976 and 1980, regardless of snail presence [6]. In high transmission areas of this valley, yearly incidence dropped from 15% to 8% and prevalence from 43% to 22% among children under ten years old [1, p.270]. Researchers concluded that, although more limited in scope, routine focal snail control was sufficient for decreasing infection levels, and they re- commended continuing this intervention at 6-week intervals for the foreseeable future [7–10,] (Fig 2).
In 1978, Project researchers began a trial of supplementary biological control of the inter- mediate host B. glabrata snails by means of introducing non-native competitor snails such as Helisoma duryi and Melanoides tuberculata to local aquatic habitats, particularly to known transmission sites [11, 12]. Post-Project snail surveys in the late 1980s, the 1990s, and in recent years indicate that M. tuberculata has become the most common freshwater snail in St. Lucia [12]. There is now an absence or very low density of B. glabrata in many former S. mansoni transmission sites, suggesting a major shift in the snail ecology of the island [12]. This environ- mental change may explain, in part, the very low numbers of human S. mansoni infections now reported by island health service workers.
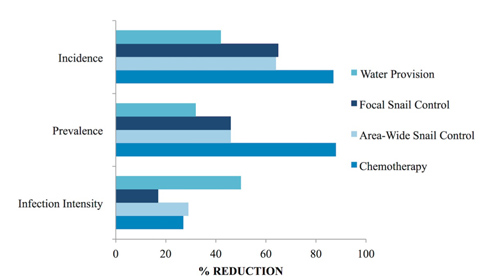 Fig 2
Fig 2
Fig 2. Comparison of the impact of individual control measures implemented during the St. Lucia study. The effects of provision of water, of focal and area-wide snail control via mollusciciding, and of targeted chemotherapy in reducing S. mansoni incidence per annum, prevalence, and mean infection intensity (for those with egg-positive stools). Relative reductions over the initial 3–5 year course of each intervention are shown (as percentages) indicated by the horizontal bars. Data from [1, pp. 270–271].
https://doi.org/10.1371/journal.pntd.0006223.g002
Follow-on crossover trials that give us our best estimates for the impact of combining interventions
Anti-schistosomal chemotherapy was arguably the most successful single intervention during the initial phase of the trials. However, after the four annual chemotherapy campaigns were completed in the Marquis Valley (1973–76), the yearly incidence of S. mansoni infection rose again in Fond Assau and Talvern areas of the valley, without a clear explanation [1, p.106]. Three years after the final treatment, their incidence had returned nearly to pre-intervention levels, and infected snails were found in streams around the Marquis Valley [1, p.115; 7]. In addition to offering chemotherapy to residents again in 1979 and 1980, the Department responded by adding focal snail control to this site in March of 1980 [1, pp.95/113]. In the last 12 months of the St. Lucia project, no more infected snails were found in Marquis Valley, suggesting that the combined approach was significantly more successful in achieving interruption of transmission [1, p.115].
In South Richefond, schistosomiasis declined after installation of the new, household level water supplies. As the project progressed, and oral oxamniquine made anti-schistosomal treatment more accessible, chemotherapy was offered to any local Richefond residents who were still infected between 1975 and 1977 [1, p.199]. The household water plus treatment approach was seen by the Department as a way of strengthening the effects of chemotherapy because the household water-related reductions in yearly incidence of S. mansoni infection meant that people were less likely to become immediately re-infected after treatment [1, p.199]. Interestingly, a temporary resurgence in infections was seen when community members, interpreting their reported low incidence as meaning their rivers were safe, returned to the still-infested waters and yearly incidence again increased to pre-control levels [1, p.199]. This added chemotherapy campaign was carried out for 3 years, and afterwards, no further intervention was done other than maintaining the relatively costly water systems [1, pp.199/207]. By the end of the study in 1981, following this combination of interventions, annual incidence among children was at only 1% and prevalence was at 9% [1, p.271]
In Cul de Sac Valley, after the initial 1970–75 area-wide snail control program, the research team launched two further years of focal snail surveillance and control [1, p.152; 8], combined with targeted chemotherapy [1, p.154; 9]. This combined intervention was successful and further reduced prevalence from 24% to 6% [1, p.271]. Subsequently, the Department chose 12 streams and 26 banana drains (out of 145 initial sites) to undergo routine mollusciciding every four weeks regardless of observed snail levels [10]. This regimen continued for the last four years of the St. Lucia project and was sufficient to prevent any resurgence of infection [10].
Importance of reviewing these outcomes in light of upcoming plans for revisions to WHO guidelines for schistosomiasis control
With good reason, mass drug administration is now the mainstay of schistosomiasis morbidity control. But when chemotherapy fails to prevent reinfection, alternative control methods must be considered to interrupt transmission. The Marquis Valley chemotherapy trial was significantly enhanced when supplemented with snail control, and Cul de Sac and South Richefond obtained impressive results by supplementing snail control or WASH-related measures with chemotherapy. The St. Lucia study was not a randomized trial, and very local factors may have biased some of its findings. However, its extensive observational data on the concurrent implementation of very different approaches to schistosomiasis control offers a very strong base of evidence for modern-day decision-making regarding potential elimination strategies. The project’s results are not fully applicable to every country fighting schistosomiasis, particularly given St. Lucia’s island ecology and the fact that the study only dealt with S. mansoni. Nevertheless, the island has significantly minimized Schistosoma infections, and the results of the multiple approaches that it undertook back then deserve a close second look today.
Supporting information
S1 File. Listing of St. Lucia Project research papers 1963–1993. This document file (.docx) provides an alphabetical listing, by author, of 133 published research papers related to the St. Lucia Project.
(DOCX)
S2 File. St. Lucia Project research papers in EndNote format (.enlx). This searchable data- base file contains information on 133 published research papers related to the St. Lucia Project and their related citation meta-data in EndNote file format (.enl plus supplemental files in a compressed.zip file).
(ZIP)
Acknowledgments
The authors would like to acknowledge the late Dr. Peter ‘Pip’ Jordan, and the late Dr. Bob Sturrock for their many past helpful discussions about the design and performance of the St. Lucia Project.
References
1. Jordan P. Schistosomiasis: The St. Lucia Project. Cambridge: Cambridge University Press; 1985.
2. Cook JA, Jordan P, Woodstock L, Pilgrim V. A controlled trial of hycanthone and placebo in schistoso- miasis mansoni in St. Lucia. Ann Trop Med Parasitol. 1977; 71(2):197–202. PMID: 326208
3. Jordan P, Bartholomew RK, Grist E, Auguste E. Evaluation of chemotherapy in the control of Schist soma mansoni in Marquis Valley, Saint Lucia. I. Results in humans. Am J Trop Med Hyg. 1982; 31 (1):103–10. PMID: 7058971
4. Cook JA, Jordan P, Bartholomew RK. Control of Schistosoma mansoni transmission by chemotherapy in St. Lucia. I. Results in man. Am J Trop Med Hyg. 1977; 26(5 Pt 1):887–93.
5. Unrau GO. Individual household water supplies as a control measure against Schistosoma mansoni: A study in rural St Lucia. Bull World Health Organ. 1975; 52(1):1–8. PMID: 1082378
6. Prentice MA, Jordan P, Bartholomew RK, Grist E. Reduction in transmission of Schistosoma mansoni by a four-year focal mollusciciding programme against Biomphalaria glabrata in Saint Lucia. Trans R Soc Trop Med Hyg. 1981; 75(6):789–98. PMID: 7330940
7. Prentice MA, Barnish G. Snail infections following chemotherapy of Schistosoma mansoni in St. Lucia, West Indies. Trans R Soc Trop Med Hyg. 1981; 75(5):713–4. PMID: 7330926
8. Barnish G, Christie JD, Prentice MA. Schistosoma mansoni control in Cul de Sac valley, Saint Lucia. I. A two-year focal surveillance-mollusciciding programme for the control of Biomphalaria glabrata. Trans R Soc Trop Med Hyg. 1980; 74(4):488–92. PMID: 7445045
9. Jordan P, Cook JA, Bartholomew RK, Grist E, Auguste E. Schistosoma mansoni control in Cul de Sac Valley, Saint Lucia. II. Chemotherapy as a supplement to a focal mollusciciding programme. Trans R Soc Trop Med Hyg. 1980; 74(4):493–500. PMID: 7445046
10. Barnish G, Jordan P, Bartholomew RK, Grist E. Routine focal mollusciciding after chemotherapy to con- trol Schistosoma mansoni in Cul de Sac valley, Saint Lucia. Trans R Soc Trop Med Hyg. 1982; 76 (5):602–9. PMID: 7179412
11. Jordan P, Christie JD, Unrau GO. Schistosomiasis transmission with particular reference to possible ecological and biological methods of control. A review. Acta Trop. 1980; 37(2):95–135. PMID: 6106355
12. Pointier JP. The introduction of Melanoides tuberculata (Mollusca: Thiaridae) to the island of Saint Lucia (West Indies) and its role in the decline of Biomphalaria glabrata, the snail intermediate host of Schistosoma mansoni. Acta Trop. 1993; 54(1):13–18. PMID: 8103624
Citation: Ivy JA, King CH, Cook JA, Colley DG (2018) Historical perspective: Revisiting the
St. Lucia Project, a multi-year comparison trial of schistosomiasis control strategies. PLoS Negl Trop Dis 12(1): e0006223. https://doi.org/10.1371/ journal.pntd.0006223
Editor: Darren J. Gray, Australian National University, AUSTRALIA
Published: January 31, 2018
Copyright: © 2018 Ivy et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Funding: This work received financial support from the University of Georgia Research Foundation, Inc. which was funded by the Bill & Melinda Gates Foundation for the SCORE Project. The funders had no role in planning, analysis, decision to publish, or preparation of the manuscript.
Competing interests: The authors have declared that no competing interests exist.
Note about co-author of this article, Joseph A. Cook
Co-author of this article, Joseph A. Cook, MD, MPH, DACP, is Former Executive Director of International Trachoma Initiative and Adjunct Epidemiology Professor, Gillings School for Global Public Health, University of North Carolina, Chapel Hill.
Dr. Cook is co-author with Silvio P. Mariotti, MD, Ophthalmologist, Senior Medical Office, Prevention of Blindness and Deafness, World Health Organization, of the chapter on “Trachoma” in Water and Sanitation Related Diseases and the Environment: Challenges, Interventions and Preventive Measures. Dr. Cook also co-authored the chapter “Trachoma” for the 2nd volume, entitled Water and Sanitation-Related Diseases and the Changing Environment: Challenges, Interventions, and Preventive Measures. For the 2nd Volume, his co-authors were Emma Harding-Esch, MPhil, MSc, Ph.D., Associate Professor at London School of Hygiene and Tropical Medicine, Prof David Mabey DM FRCP FMedSci, Professor of Communicable Diseases at London School of Hygiene and Tropical Medicine, and Dr Anthony Solomon MBBS, DTM&H, PhD, PGCAP, FHEA, FRCP, Medical Officer, Neglected Tropical Diseases, Department of Control of Neglected Tropical Diseases, World Health Organization.
The 2 Volumes of Water and Sanitation-Related Diseases and the Changing Environment: Challenges, Interventions, and Preventive Measures , which augment one another, were published by Wiley in collaboration with Horizon International.
Seventy-five experts from Johns Hopkins Bloomberg School of Public Health, Harvard, Yale, and countries all over the world have contributed their time and effort to make these books “a thing of beauty….” As the back cover of this new volume summarizes: “Augmenting authoritative interdisciplinary coverage in the first edition, this new edition of … expands upon the significance of the changing environment to disease vectors, food systems and nutrition, and population, and the importance of ecosystem health to human health. Many chapters stand as they are in first edition to which readers are referred, and which are not included in this volume. The books were written by … experts from the fields of climate change, environmental engineering, environmental health, epidemiology, food and agriculture, global health, medicine, medical anthropology, nutrition, population, and public health.”
Dr. Paul Farmer wrote the Foreword. This is an excerpt: “The work of experienced scholars, public‐health advocates, and implementers, this new edition …offers a thorough review of some of the ranking problems of our time. Taken individually, these chapters constitute a critical compendium of ongoing debates among experts and a concise summary of more settled matters. But editor Janine Selendy has also woven these diverse chapters—which include highly focused considerations of specific waterborne illnesses and more broad‐ranging matters from climate change to technological innovation—into a powerful and hefty manual to guide collective action going forward…. It will be an authoritative reference for practitioners and trainees to deliver on the promise of water, sanitation, and health for all.” Paul Farmer, MD, PhD Kolokotrones University Professor, Harvard University
1. Pascal Magnussen, Senior Researcher, MD, DTM&H, Specialist in Tropical Medicine and Infectious Diseases, Dept. of Immunology and Microbiology, Centre for Medical Parasitology & Dept. of Veterinary and Animal Sciences, Section for Parasitology and Aquatic Pathobiology, Faculty of Health and Medical Sciences, University of Copenhagen
2. Birgitte Jyding Vennervald, Professor, M.D., Dept. of Veterinary and Animal Sciences, Section for Parasitology and Aquatic Pathobiology, Faculty of Health and Medical Sciences, University of Copenhagen
3. Jens Aagaard-Hansen, M.D., M.P.H., Diploma T.M., and Specialist in General Medicine. Steno Diabetes Center Copenhagen, Copenhagen, Denmark & MRC Developmental Pathways for Health Research Unit, Department of Paediatrics, School of Clinical Medicine, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa.
Search
Latest articles
Agriculture
- World Water Week: Healthy ecosystems essential to human health: from coronavirus to malnutrition Online session Wednesday 24 August 17:00-18:20
- World Water Week: Healthy ecosystems essential to human health: from coronavirus to malnutrition Online session Wednesday 24 August 17:00-18:20
Air Pollution
- "Water and Sanitation-Related Diseases and the Changing Environment: Challenges, Interventions, and Preventive Measures" Volume 2 Is Now Available
- Global Innovation Exchange Co-Created by Horizon International, USAID, Bill and Melinda Gates Foundation and Others
Biodiversity
- It is time for international mobilization against climate change
- World Water Week: Healthy ecosystems essential to human health: from coronavirus to malnutrition Online session Wednesday 24 August 17:00-18:20
Desertification
- World Water Week: Healthy ecosystems essential to human health: from coronavirus to malnutrition Online session Wednesday 24 August 17:00-18:20
- UN Food Systems Summit Receives Over 1,200 Ideas to Help Meet Sustainable Development Goals
Endangered Species
- Mangrove Action Project Collaborates to Restore and Preserve Mangrove Ecosystems
- Coral Research in Palau offers a “Glimmer of Hope”
Energy
- Global Innovation Exchange Co-Created by Horizon International, USAID, Bill and Melinda Gates Foundation and Others
- Wildlife Preservation in Southeast Nova Scotia
Exhibits
- Global Innovation Exchange Co-Created by Horizon International, USAID, Bill and Melinda Gates Foundation and Others
- Coral Reefs
Forests
- NASA Satellites Reveal Major Shifts in Global Freshwater Updated June 2020
- Global Innovation Exchange Co-Created by Horizon International, USAID, Bill and Melinda Gates Foundation and Others
Global Climate Change
- It is time for international mobilization against climate change
- It is time for international mobilization against climate change
Global Health
- World Water Week: Healthy ecosystems essential to human health: from coronavirus to malnutrition Online session Wednesday 24 August 17:00-18:20
- More than 400 schoolgirls, family and teachers rescued from Afghanistan by small coalition
Industry
- "Water and Sanitation-Related Diseases and the Changing Environment: Challenges, Interventions, and Preventive Measures" Volume 2 Is Now Available
- Global Innovation Exchange Co-Created by Horizon International, USAID, Bill and Melinda Gates Foundation and Others
Natural Disaster Relief
- STOP ATTACKS ON HEALTH CARE IN UKRAINE
- Global Innovation Exchange Co-Created by Horizon International, USAID, Bill and Melinda Gates Foundation and Others
News and Special Reports
- World Water Week: Healthy ecosystems essential to human health: from coronavirus to malnutrition Online session Wednesday 24 August 17:00-18:20
- STOP ATTACKS ON HEALTH CARE IN UKRAINE
Oceans, Coral Reefs
- World Water Week: Healthy ecosystems essential to human health: from coronavirus to malnutrition Online session Wednesday 24 August 17:00-18:20
- Mangrove Action Project Collaborates to Restore and Preserve Mangrove Ecosystems
Pollution
- Zakaria Ouedraogo of Burkina Faso Produces Film “Nzoue Fiyen: Water Not Drinkable”
- "Water and Sanitation-Related Diseases and the Changing Environment: Challenges, Interventions, and Preventive Measures" Volume 2 Is Now Available
Population
- "Water and Sanitation-Related Diseases and the Changing Environment: Challenges, Interventions, and Preventive Measures" Volume 2 Is Now Available
- "Water and Sanitation-Related Diseases and the Changing Environment: Challenges, Interventions, and Preventive Measures" Volume 2 Is Now Available
Public Health
- Honouring the visionary behind India’s sanitation revolution
- Honouring the visionary behind India’s sanitation revolution
Rivers
- World Water Week: Healthy ecosystems essential to human health: from coronavirus to malnutrition Online session Wednesday 24 August 17:00-18:20
- Mangrove Action Project Collaborates to Restore and Preserve Mangrove Ecosystems
Sanitation
- Honouring the visionary behind India’s sanitation revolution
- Honouring the visionary behind India’s sanitation revolution
Toxic Chemicals
- "Water and Sanitation-Related Diseases and the Changing Environment: Challenges, Interventions, and Preventive Measures" Volume 2 Is Now Available
- Actions to Prevent Polluted Drinking Water in the United States
Transportation
- "Water and Sanitation-Related Diseases and the Changing Environment: Challenges, Interventions, and Preventive Measures" Volume 2 Is Now Available
- Urbanization Provides Opportunities for Transition to a Green Economy, Says New Report
Waste Management
- Honouring the visionary behind India’s sanitation revolution
- Honouring the visionary behind India’s sanitation revolution
Water
- Honouring the visionary behind India’s sanitation revolution
- Honouring the visionary behind India’s sanitation revolution
Water and Sanitation
- Honouring the visionary behind India’s sanitation revolution
- Honouring the visionary behind India’s sanitation revolution

