Navigation
Chinese Scientists Show That Intercropping Maize With Faba Beans Increases Yield
Intercropping, which grows at least two crop species on the same pieces of land at the same time, can increase grain yields greatly.
The following is an open-access PNAS (Proceedings of the National Academy of Sciences of the United States of America) article.
 |
| Vicia faba plants in flower Photograph: Wikipedia |
By: Long Li*, , Shu-Min Li , Jian-Hao Sun , Li-Li Zhou*, Xing-Guo Bao , Hong-Gang Zhang*, and Fu-Suo Zhang*,
Abstract
Intercropping, which grows at least two crop species on the same pieces of land at the same time, can increase grain yields greatly. Legume–grass intercrops are known to overyield because of legume nitrogen fixation. However, many agricultural soils are deficient in phosphorus. Here we show that a new mechanism of overyielding, in which phosphorus mobilized by one crop species increases the growth of a second crop species grown in alternate rows, led to large yield increases on phosphorus-deficient soils. In 4 years of field experiments, maize (Zea mays L.) overyielded by 43% and faba bean (Vicia faba L.) overyielded by 26% when intercropped on a low-phosphorus but high-nitrogen soil. We found that overyielding of maize was attributable to below-ground interactions between faba bean and maize in another field experiment. Intercropping with faba bean improved maize grain yield significantly and above-ground biomass marginally significantly, compared with maize grown with wheat, at lower rates of P fertilizer application (<75 kg of P2O5 per hectare), and not significantly at high rate of P application (>112.5 kg of P2O5 per hectare). By using permeable and impermeable root barriers, we found that maize overyielding resulted from its uptake of phosphorus mobilized by the acidification of the rhizosphere via faba bean root release of organic acids and protons. Faba bean overyielded because its growth season and rooting depth differed from maize. The large increase in yields from intercropping on low-phosphorus soils is likely to be especially important on heavily weathered soils.
Intercropping, which is the intermingled growth of two or more crops, with >28 million hectares of annually sown area in China (1), is also common in other parts of the world, such as in India, Southeast Asia, Latin America, and Africa (2). On nitrogen-deficient soils, legume–grass intercrops are known to overyield because of legume nitrogen fixation (2–5). About a third of terrestrial soils have insufficient available phosphorus (P) for optimum crop production, with many tropical acid soils being highly P-deficient (6, 7). Some pot experiments have suggested that legume/cereal mixtures can achieve greater P uptake on such soils (8–10) than can either species by itself. In field conditions, similar greater P uptake by intercropped maize with faba bean also was observed (11). However, both pot experiments and field experiments did not distinguish that the greater P uptake was derived from niche (rooting depth or seasonality) complementary or direct interspecific facilitation. We hypothesize that overyielding of intercropped species on P-deficient soil may result from a plant's chemical alteration of the rhizosphere that mobilizes P and thus enhances its own productivity and that of another species. We call this phenomenon the interspecific rhizosphere effect. Such chemical mobilization of a limiting nutrient would represent a mechanism of interspecific facilitation that, in concert with interspecific differences in seasonality (cool/warm) and rooting depth, could play an important role in meeting escalating global demand, especially in tropical habitats.
Results and Discussion |
We performed a 4-yr field experiment (Field Study 1) in which maize and faba bean were either planted as monoculture crops or were planted in alternating rows (intercropped) in an agricultural site in which P is the major limiting soil nutrient. A high rate of N fertilization [N at 225 kg·hectare (ha)–1·yr–1] further assured that P, but not N, would limit yields. We found that, when intercropped, maize grain overyield by 43% (range: 17–74%) (P < 0.0001) and faba beans overyield by 26% (range: 3–33%) (P = 0.0010), compared with corresponding monocultured maize and faba bean, on average over the 4 yr [Table 1 and supporting information (SI) Table 4]. There was a stronger and more consistent positive effect of intercropping on maize than on faba bean. Year-by-year analyses of results showed that maize, but not faba bean, significantly overyielded each of the 4 yr (SI Table 4). Similar results also were observed for above-ground biomass production (Table 1 and SI Table 4).
|
In another field experiment in which rows of maize and faba bean were grown either with no root barrier, with a mesh barrier, or with a solid barrier (Field Study 2), we found that overyielding of maize was attributable to below-ground interactions between faba bean and maize. Both grain yield and above-ground biomass of maize and faba bean without root barrier (below-ground interactions) were significantly (P < 0.0001 for maize grain yield and P = 0.0003 for faba bean grain yield, and P < 0.0001 and P = 0.006 for biomass of maize and faba bean, respectively) greater than those of faba bean and maize with root barriers (without below-ground interactions) (Table 2).
|
We also found that, in Field Study 3 with 225 kg/ha N of fertilization, intercropping with faba bean improved maize grain yield significantly (P < 0.04) or above-ground biomass marginally significantly (P < 0.08), compared with maize grown with wheat, at lower rates of P fertilizer application (0, 37.5, and 75 kg/ha P2O5), but the difference was no longer significant (P = 0.2386–0.8991) when P supply was sufficient (112.5 and 150 kg/ha P2O5) (Table 3). These findings indicate that, on these P-deficient soils, a P nutrition improvement in faba bean/maize intercropping played an important role in the overyielding of maize through interspecific interactions between faba bean and maize.
|
To study the causes of these observed yield effects, we grew maize and faba bean in greenhouse conditions in three types of pots and in various solubilities of P forms that are common in soils (Greenhouse Study 1). We used root barriers to measure interspecific rhizosphere effects. In one treatment, a 30-µm nylon mesh barrier divided pots in half, with a plant of each species planted in each half. The nylon mesh barrier blocked root growth into the other half but permitted exchange of root exudates. In the second treatment, a plastic sheet (solid) root barrier eliminated any interspecific rhizosphere effect by preventing root exudates from moving between two species root zones, whereas the same soil volume occupied by individual plant species as the treatment with the mesh root barrier. The third treatment had no root barrier (methods). If there is significantly greater biomass with a mesh barrier than with a solid barrier, this would be evidence of an interspecific rhizosphere effect.
We observed that the biomass of maize (including roots and shoots) was significantly greater (F1, 27 = 71.81, P < 0.0001) with a mesh barrier than with a solid barrier, on average for all P source treatments in Greenhouse Study 1. Furthermore, the biomass of maize with a mesh barrier was greater than that with solid barrier under addition of Ca(H2PO4)2-P (Ca-P, a highly soluble form of P) (P = 0.0128), FePO4-P [Fe-P, an insoluble form of P, Ksp (25°C) = 9.91 x 10–16] (P < 0.0001), AlPO4-P [Al-P, the least soluble form of P that we used, Ksp (25°C) = 9.84 x 10–21] (P = 0.0007), and without P addition (P = 0.0014), but not under addition of organic P (OP, inositol hexaphosphate) (P = 0.4370). The interspecific rhizosphere effect did not impact significantly the biomass of faba bean (F1, 27 = 0.31, P = 0.5816).
These results indicate that overyielding of maize, a crop species that has a high requirement for P, resulted from a rhizosphere effect of faba bean on maize, especially when P was provided in an insoluble form, such as AlPO4- and FePO4-P (Fig. 1a). In the root separation field study, the biomass of maize, when its roots were separated from faba bean by nylon mesh, was significantly greater than that of maize when its roots were separated from faba bean by a plastic sheet (4). In this Greenhouse Study 1, we eliminated seasonality or rooting space niche complementary effects by root barriers, where two plant species occupied the same soil space (solid root barrier versus nylon mesh barrier) and were planted and harvested at the same time. Therefore, we demonstrated that an interspecific rhizosphere effect indeed played an important role in the interspecific facilitation between intercropped species. In this study, a nitrogen fixation effect, and effects of all other nutrients except for P were eliminated by addition of 200 mg of N per kilogram of soil as NH4NO3 and sufficient other nutrients (see Methods).
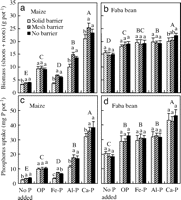 |
| Fig. 1. Biomass (dry weight of shoots plus roots) of maize (a), faba bean (b), and P uptake by maize (c) and faba bean (d) supplied with different P forms and grown in various treatments of root barrier between two species for 74 days (Greenhouse Study 1). Bar groups with different capital letters indicate a significant difference (P < 0.05) between forms of phosphorus treatment. Bars with different lowercase letters indicate a significant difference (P < 0.05) among three treatments of root interactions within the same phosphorus form or source. Error bar is a SD (n = 4) (Greenhouse Study 1). |
View larger version (41K):
[in this window]
[in a new window]
Biomass of maize was significantly influenced by the P source (F4, 27 = 501, P < 0.0001), indicating that maize has considerable variation in acquiring insoluble P in soil. In contrast, faba bean biomass did not depend on the P source (Ca-P, Al-P, and Fe-P) (F2, 15 = 1.11, P = 0.3560), indicating that faba bean can use many forms of soil inorganic P.
The interspecific rhizosphere effect significantly increased P uptake by maize on average for all P sources, compared with uptake without a rhizosphere effect (F1, 27 = 43.19, P < 0.0001). Phosphorus uptake by maize with a rhizosphere connection (mesh barrier) was 30% greater than that without an interspecific rhizosphere connection (solid barrier) for pots without any P addition (P < 0.0033), 116% greater for pots with Fe-P addition (P < 0.0001), 56.1% greater for pots with Al-P (P = 0.0009), and 12% greater for pots with Ca-P (P = 0.0710) (Fig. 1c). In contrast, there were no significant differences in P uptake by faba bean between the mesh barrier and the solid barrier (Fig. 1d). The results indicate that faba bean facilitated P uptake by maize of otherwise sparingly soluble inorganic P sources. In the experiment, a significant linear relationship between shoot biomass [DM (gram per pot)] and shoot P concentration [Pconc (g·kg–1)] in maize was observed (DM = 14.699 x Pconc – 9.2907, R2 = 0.7784, n = 60), further suggesting that the difference in maize growth for various root barrier and P addition treatments was related to P nutrition.
We observed that faba bean acidified its rhizosphere intensively, with pH declining 2 units in agar gel in 6 h (Fig. 2a) in Greenhouse Study 2. In contrast, maize alkalized its rhizosphere (Fig. 2c). A decrease in the soil pH from 6.5 to 4.1 could result in at least a 10-fold increase in the P released into solution (12), which could explain the greater P uptake by maize in our results.
 |
| Fig. 2. Visualization of rhizosphere acidification of faba bean (a), soybean (b), and maize (c). The roots were imbedded for 6 h in agar gel containing a pH indicator (bromocresol purple) without P supply (Greenhouse Study 2). Yellow indicates acidification, and purple indicates alkalization. |
View larger version (70K):
[in this window]
[in a new window]
We also found that both malate and citrate concentrations in the rhizosphere soil of faba bean were significantly greater (P < 0.0001 for malate and P = 0.0014 for citrate) than those in maize in Greenhouse Study 3 (Fig. 3). Other work has shown that, compared with monocots, dicots, particularly legumes, produced and excreted more organic acids to the rhizosphere, which enhanced P solubility (13). For instance, piscidic acid exuded from the roots of pigeon pea promoted the release of P from FePO4 by chelating iron (9). Citric and malic acids are the major components of root exudates of lupine and play an important role in P uptake in P deficient soil (14). Under organic P (inositol hexaphosphate) supply, chickpea has been found to improve P nutrition of wheat in a similarly designed pot experiment (15) because of acid phosphatase activity in its rhizosphere (16). These studies showed that a plant releasing more protons, organic acids, or phosphatase mobilized more inorganic P or organic P in soil (9, 13, 15, 17), which benefits itself and other plant species whose roots are intermingled in a diverse community of plants.
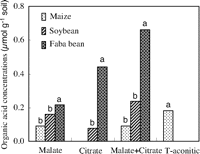 |
| Fig. 3. Concentration of malate and citrate in the rhizosphere of maize, soybean, and faba bean. Harvests were at 35 and 45 days after emergence, respectively. Data from two samplings were combined into one data set as replicates because no significant difference was found between the two samplings. Differences among the letters above the bars indicate a significant (P < 0.05) difference in organic acids between crops (n = 11) (Greenhouse Study 3). |
View larger version (22K):
[in this window]
[in a new window]
Faba bean overyielded when intercropped with maize in field conditions (Table 1 and SI Table 4) but not in pots (Fig. 1b). This probably was a result of seasonality and rooting niche differentiation effects, because, under field conditions, faba bean grew and developed earlier than maize, so that its roots could spread into below-ground space under maize (18).
Our findings provide an additional reason for the frequently observed positive effects of plant species number on ecosystem productivity (19–24), especially in the absence of nitrogen fixation for legumes. Previous studies were unable to distinguish niche differentiation (seasonality or rooting depth) effect from direct interspecific facilitation (20, 22, 25). Our results present an example of direct interspecific facilitation. Interspecific rhizosphere effects may be a biologically significant mechanism for a positive relationship between plant diversity and productivity.
Intercropping has been practiced by farmers in China for >2,000 yr. Recent research shows that intercropping can decrease yields by interspecific competition or increase them up to 89% by reducing limitation of crop growth by nitrogen (2, 3, 5), phosphorus and/or disease (26). Because global demand for food is projected to double in the coming 50 years (27), the greater yields and efficient land utilization from intercropping could be a significant factor in meeting escalating global food demand if intercropping were to be adopted in other regions. On the other hand, high yields can be obtained from P-efficient species/P-inefficient species intercropping without P fertilizer, which means that P fertilizer, a limited resource (7), can be conserved and farmers can spare the expense of purchasing fertilizer. In addition, P fertilizer is less likely to contaminate surface waters through intercropping. However, intercropping on a global scale would require the development of agricultural machinery and techniques appropriate to intercropping, because agriculture in all developing nations is becoming increasingly more mechanized.
Conclusions |
Our data show that intercropping of maize and faba bean leads to marked overyielding and support the hypothesis that one of the mechanisms behind intercropping facilitation on a P deficient soil is an interspecific rhizosphere effect on P nutrition because (i) rhizosphere acidification by P-efficient species resulted in a pH decrease in the rhizosphere, which increased the availability of insoluble inorganic P in soil (7, 12), such as FePO4 and AlPO4; (ii) carboxylates from root exudation of one species chelated Ca, Fe, and Al, consequently mobilizing insoluble soil P (17), which will benefit the species and other species grown together with it; and (iii) greater phosphatase activity in the rhizosphere decomposed soil organic P into an inorganic form, which can be used by both species, such as wheat/chickpea and maize/chickpea (15, 16).
Methods |
Field Study 1. Field Study 1 was conducted in 2003 at the Baiyun Experimental site (28) located in Western Gansu Province, China. Experimental treatments were continuously cropped maize; continuously cropped faba bean; faba bean (four rows) and maize (two rows) continuously intercropped on the same strips of land; and four rows of faba bean and two rows of maize rotationally intercropped with one crop strips in 1 yr and the other crop strips in subsequent year. One intercropping combination included a 0.8-m faba bean strip (four rows of faba bean, with a 0.2-m inter-row distance) and a 0.8-m maize strip (two rows of maize with a 0.4-m inter-row distance), so that two crop strips can be exchanged in a subsequent year for the rotaionally intercropped maize/faba bean treatment. Interplant distance within the same row was 0.2 m for faba bean and 0.25 m for maize in intercropping and monocropping. Inter-row distance in monocropping was 0.20 m for faba bean and 0.40 m for maize, which made the planting density in monocropping identical to intercropping in a comparable area. Each treatment had three replicates, and all of plots received 225 kg/ha N fertilizer and 40 kg/ha P2O5 fertilizer. The individual plot area was 8.0 x 5.6 m, and a ridge (with a width of 0.4 m and a height of 0.3 m) between adjacent plots was constructed to separate the plots. There were five combinations on individual intercropping plot. To prevent water stress, all plots were irrigated six to seven times according to the normal on-farm practice in this area. The biomass and grain yields were based on comparable area, i.e., actually occupied area by one species in intercropping versus an identical area in monocropping.
Field Study 2. For Field Study 2, we grew rows of maize and faba bean with roots separated by either a solid or mesh root barrier, or we grew them with no root barrier. This experiment was conducted in 2004 at the same site as Field Study 1, using a split-split-plot design. The main plot was treated with rhizobium inoculation or not; the subplot was treated with arbuscular mycorrhizal fungal inoculation or not, the sub-subplot was treated with one of three root barriers (solid, mesh, and without root barrier) between faba bean and maize roots. Each treatment had four replicates. Because of soil disturbance resulting from root barrier installation, only 2005 (second year) results are presented. Yield and biomass of both crops did not respond to either rhizobium inoculation or arbuscular mycorrhizal fungal inoculation, although both were without N and P fertilization. Thus, we combined both inoculation treatments as replications (n = 16) of root barrier treatment.
Field Study 3. Field Study 3 was conducted in 2000 at the same site as Field Study 1, with split-plot design. The main plot treatment was P application rates at 0, 37.5, 75, 112.5 or 150 kg/ha P2O5, and the subplot treatment was either wheat/maize intercropping [six rows of wheat (0.7-m wheat strip, with a 0.12-m inter-row distance) were intercropped with two rows of maize (0.8-m maize strip, with a 0.40-m inter-row distance)] or faba bean/maize [two rows of faba bean (0.4-m faba bean strip, with a 0.2-m inter-row distance) were intercropped with two rows of maize (0.8-m maize strip, with a 0.4-m inter-row distance)]. Each treatment had three replicates. All plots received N fertilizer at 225 kg/ha to eliminate N limitation.
Greenhouse Study 1. We used pots (0.15 m in diameter, 0.15 m in depth) with two compartments to provide three types of root interactions, including a solid barrier to eliminate root contact and solute movement, a nylon mesh (30 µm) barrier to prevent root intermingling of two species but permit root exudate exchange, and no root barrier. Plastic pots were cut in the middle, separated by the appropriate material into two compartments, and then reconstructed. One of four P sources was added as powder before mixing at 60 mg of P per kilogram of soil: inositol hexaphosphate-Na (OP); Ca(H2PO4)2·H2O (Ca-P, highly water soluble P); FePO4 [Fe-P, an insoluble form of P, Ksp (25°C) = 9.91 x 10–16]; AlPO4 [Al-P, the least soluble form of P that we used, Ksp (25°C) = 9.84 x 10–21], a control with no P addition was also included. There were 15 treatments with four replications. The soil was a low-P sandy soil with pH 7.8. Basal nutrients were added in solution (as milligrams per kilogram of soil): N, 200 mg as NH4NO3; K, 200 mg as KNO3; Mg, 50 mg as MgSO4·7H2O; Fe, 5 mg as FeSO4·7H2O; Mn, 5 mg as MnSO4; Cu, 5 mg as CuSO4·5H2O; and Zn, 5 mg as ZnSO4·7H2O.
Greenhouse Study 2. Germinated seeds were placed on 28-cm-long and 20-cm-wide filter paper, where nylon mesh (30 µm) was placed beneath to keep the roots from penetrating the filter paper. An additional layer of filter paper and nylon mesh were added on top. The filter papers and nylon mesh layers were fixed by using two parallel clear plastic plates and two elastic clips. The nutrient solution contained the following minerals: 0.75 x 10–3 mol/liter K2SO4, 0.65 x 10–3 mol/liter MgSO4, 0.1 x 10–3 mol/liter KCl, 2.0 x 10–3 mol/liter Ca(NO3)2, 0.20 x 10–3 mol/liter KH2PO4, 1 x 10–6 mol/liter H3BO3, 1 x 10–6 mol/liter MnSO4, 1 x 10–7 mol/liter CuSO4, 1 x 10–6 mol/liter ZnSO4, 5 x 10–9 mol/liter (NH4)6Mo2O4, and 1 x 10–4 mol/liter Fe-EDTA. The pH of the nutrient solution was adjusted to 6.0 (i.e., initial pH of the solution was 6.0) by adding 0.01 mol/liter HCl or 0.01 mol/liter NaOH. The nutrient solution was renewed every 3 days. The difference in rhizosphere acidification potential was studied visually and by quantifying proton efflux of roots (29) between faba bean (Vicia faba L. cv. Lincan No. 5), soybean (Glycine max L. Zhonghuang No. 17), and maize (Zea mays L. cv. Zhongdan No. 2), supplied with or without 0.2 mmol/liter P as KH2PO4. After grown for 14 days in treatment solution, the intact plants were carefully taken from the nylon mesh and washed in 0.2 mmol/liter CaSO4 solution for a few minutes and then rinsed in distilled water with pH 5.5. Afterward, the whole plant roots were placed on a 3-mm-thick agar gel (9.0 g/liter) film with pH 5.5 containing 0.1 g/liter pH indicator (bromocresol purple) and complete nutrient solution without P addition and with P supplied at 0.2 mmol/liter. To separate the root, we used a nipper and a transparent film and embedded three-quarters of the root surface in the agar. Then we removed the transparent film and used a glass plate to place the agar gel film 3 mm above the surface to provide aeration to the root. The agar gel film was wrapped with aluminum foil to avoid light penetration into the root zone and placed at a 45° angle for 6 h.
Greenhouse Study 3. We used the same design as Greenhouse Study 1 to grow faba bean, soybean, and maize in sandy soil for 35 and 45 days. All nutrients were added to the soil, similar to Greenhouse Study 1, except that no P was added. At harvest, root systems were held by the stem base and gently lifted out of the soil. The roots were carefully shaken to remove excess soil, and clumps of soil trapped between roots were taken out. The soil that remained adhering to the roots was defined as rhizosphere soil. The root system was transferred to a beaker, and rhizosphere soil was washed off the roots by adding 0.2 mM CaCl2 and shaking the beaker gently. Organic acids were measured by using HPLC according to the methods of Woulterlood et al. (30). There were 11 replicates with each species: six from the first sampling (35 d), and five from the second sampling (45 d). There were no significant differences between the two samplings.
Acknowledgements |
Top |
We thank D. Tilman, D. Fornara, C. Clark, and anonymous reviewers for comments and suggestions that improved the manuscript. This work was supported by the National Natural Science Foundation of China, the National Basic Research Program of China, and Program for Changjiang Scholars and Innovative Research Team in University Project IRT0511.
Footnotes |
Abbreviations: ha, hectare.
To whom correspondence may be addressed at: College of Resources and Environmental Sciences, China Agricultural University, 2 Yuan Ming Yuan West Road, Beijing 100094, China. E-mail: lilong@cau.edu.cn or zhangfs@cau.edu.cn
Freely available online through the PNAS open access option.
Author contributions: L.L. and F.-S.Z. designed research; S.-M.L., J.-H.S., L.-L.Z., X.-G.B., and H.-G.Z. performed research; L.L., S.-M.L., L.-L.Z., and H.-G.Z. analyzed data; and L.L. wrote the paper.
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0704591104/DC1.
© 2007 by The National Academy of Sciences of the USA
References |
- Liu, XH. (1994) The Farming Systems (China Agric Univ Press, Beijing,).
- Vandermeer, J. (1989) The Ecology of Intercropping (Cambridge Univ Press, Cambridge,).
- Stern, WR. (1993) Field Crops Res 34, 335–356.[CrossRef]
- Li, L, Yang, SC, Li, XL, Zhang, FS & Christie, P. (1999) Plant Soil 212, 205–214.
- Knudsen, MT, Hauggaard-Nielsen, H, Jornsgard, B & Jensen, ES. (2004) J Agric Sci 142, 617–627.[CrossRef]
- Batjes, NH. (1997) Soil Use Manage 13, 9–16.[CrossRef]
- Vance, CP, Uhde-Stone, C & Allan, DL. (2003) New Phytol 157, 423–447.[CrossRef][ISI]
- Horst, BG & Waschkies, C. (1987) Z Pflanzernaehr Bodenkd 150, 1–8.[CrossRef]
- Ae, N, Arihara, J, Okada, K, Yoshihara, T & Johansed, C. (1990) Science 248, 477–480.[Abstract/Free Full Text]
- EI Dessougi, H, zu Dreeele, A & Claassen, N. (2003) J Plant Nutri Soil Sci 166, 254–261.[CrossRef]
- Li, L, Zhang, FS, Li, XL, Christie, P, Yang, SC & Tang, C. (2003) Nutr Cycl Agroecosyst 65, 61–71.[CrossRef]
- Grinsted, MJ, Hedley, MJ, White, RE & Nye, PH. (1982) New Phytol 91, 19–29.[CrossRef][ISI]
- Raghothama, KG. (1999) Ann Rev Plant Physi Mol Biol 50, 665–693.[CrossRef]
- Dakora, DF & Phillips, DA. (2002) Plant Soil 245, 35–47.[CrossRef][ISI]
- Li, L, Tang, C, Rengel, Z & Zhang, FS. (2003) Plant Soil 248, 305–312.[CrossRef][ISI]
- Li, SM, Li, L, Zhang, FS & Tang, C. (2004) Ann Bot 94, 297–303.[Abstract/Free Full Text]
- Dinkelaker, B, Römheld, V & Marschner, H. (1989) Plant Cell Environ 12, 285–292.[CrossRef]
- Li, L, Sun, JH, Zhang, FS, Guo, TW, Bao, XG, Smith, AF & Smith, S. (2006) Oecologia 147, 280–290.[CrossRef][ISI][Medline]
- Tilman, D, Wedin, D & Knops, J. (1996) Nature 379, 718–720.[CrossRef]
- Tilman, D, Reich, P, Knops, J, Wedin, D, Mielke, T & Lehman, C. (2001) Science 294, 843–845.[Abstract/Free Full Text]
- Tilman, D, Hill, J & Lehman, C. (2006) Science 314, 1598–1600.[Abstract/Free Full Text]
- Hector, A, Schmid, B, Beierkuhnlein, C, Caldeira, MC, Diemer, M, Dimitrakopoulos, PG, Finn, JA, Freitas, H, Giller, PS & Good, J, et al. (1999) Science 286, 1123–1127.[Abstract/Free Full Text]
- Hooper, DU & Vitousek, PM. (1997) Science 277, 1302–1305.[Abstract/Free Full Text]
- Cardinale, BJ, Srivastava, DS, Duffy, JE, Wright, JP, Downing, AL, Sankaran, M & Jouseau, C. (2006) Nature 443, 989–992.[CrossRef][Medline]
- Loreau, M & Hector, A. (2001) Nature 412, 72–76.[CrossRef][Medline]
- Zhu, YY, Chen, H, Fan, J, Wang, Y, Li, Y, Chen, J, Fan, J, Yang, S, Hu, L & Leung, H, et al. (2000) Nature 406, 718–722.[CrossRef][Medline]
- Fedoroff, NV & Cohen, JE. (1999) Proc Natl Acad Sci USA 96, 5903–5907.[Free Full Text]
- Li, L, Sun, JH, Zhang, FS, Li, XL, Yang, SC & Rengel, Z. (2001) Field Crops Res 71, 123–137.[CrossRef]
- Rao, TP, Yano, K, Yamauchi, A & Tatsumi, J. (2000) Plant Prod Sci 3, 101–107.
- Wouterlood, M, Cawthray, GR, Scanlon, TT, Lambers, H & Veneklaas, EJ. (2004) New Phytol 162, 745–753.[CrossRef][ISI]
*Key Laboratory of Plant and Soil Interactions, Ministry of Education, College of Resources and Environmental Sciences, China Agricultural University, Beijing 100094, China; Institute of Soils and Fertilizers, Gansu Academy of Agricultural Sciences, Lanzhou 730070, China; and Resource and Environmental College, Northeast Agricultural University, Harbin 150030, China
Communicated by G. David Tilman, University of Minnesota, St. Paul, MN, May 16, 2007 (received for review February 14, 2007)
Published online before print June 25, 2007, 10.1073/pnas.0704591104
PNAS | July 3, 2007 | vol. 104 | no. 27 | 11192-11196
OPEN ACCESS ARTICLE: BIOLOGICAL SCIENCES / AGRICULTURAL SCIENCES
***************************************************************************
Note related information:
Case study on the Horizon Solutions Site: Policy Dynamics and Alley Farming Adoption in West and Central Africa
In the early 1980s, alley farming was developed as an agroforestry practice that is capable of enhancing the sustainability of small-scale farming, the activity of the vast majority of people in the region. The innovation has contributed to soil fertility, enhanced crop production and provision of fodder for animals.
TV program on Integrated Pest Management in China:
Search
Latest articles
Agriculture
- World Water Week: Healthy ecosystems essential to human health: from coronavirus to malnutrition Online session Wednesday 24 August 17:00-18:20
- World Water Week: Healthy ecosystems essential to human health: from coronavirus to malnutrition Online session Wednesday 24 August 17:00-18:20
Air Pollution
- "Water and Sanitation-Related Diseases and the Changing Environment: Challenges, Interventions, and Preventive Measures" Volume 2 Is Now Available
- Global Innovation Exchange Co-Created by Horizon International, USAID, Bill and Melinda Gates Foundation and Others
Biodiversity
- It is time for international mobilization against climate change
- World Water Week: Healthy ecosystems essential to human health: from coronavirus to malnutrition Online session Wednesday 24 August 17:00-18:20
Desertification
- World Water Week: Healthy ecosystems essential to human health: from coronavirus to malnutrition Online session Wednesday 24 August 17:00-18:20
- UN Food Systems Summit Receives Over 1,200 Ideas to Help Meet Sustainable Development Goals
Endangered Species
- Mangrove Action Project Collaborates to Restore and Preserve Mangrove Ecosystems
- Coral Research in Palau offers a “Glimmer of Hope”
Energy
- Global Innovation Exchange Co-Created by Horizon International, USAID, Bill and Melinda Gates Foundation and Others
- Wildlife Preservation in Southeast Nova Scotia
Exhibits
- Global Innovation Exchange Co-Created by Horizon International, USAID, Bill and Melinda Gates Foundation and Others
- Coral Reefs
Forests
- NASA Satellites Reveal Major Shifts in Global Freshwater Updated June 2020
- Global Innovation Exchange Co-Created by Horizon International, USAID, Bill and Melinda Gates Foundation and Others
Global Climate Change
- It is time for international mobilization against climate change
- It is time for international mobilization against climate change
Global Health
- World Water Week: Healthy ecosystems essential to human health: from coronavirus to malnutrition Online session Wednesday 24 August 17:00-18:20
- More than 400 schoolgirls, family and teachers rescued from Afghanistan by small coalition
Industry
- "Water and Sanitation-Related Diseases and the Changing Environment: Challenges, Interventions, and Preventive Measures" Volume 2 Is Now Available
- Global Innovation Exchange Co-Created by Horizon International, USAID, Bill and Melinda Gates Foundation and Others
Natural Disaster Relief
- STOP ATTACKS ON HEALTH CARE IN UKRAINE
- Global Innovation Exchange Co-Created by Horizon International, USAID, Bill and Melinda Gates Foundation and Others
News and Special Reports
- World Water Week: Healthy ecosystems essential to human health: from coronavirus to malnutrition Online session Wednesday 24 August 17:00-18:20
- STOP ATTACKS ON HEALTH CARE IN UKRAINE
Oceans, Coral Reefs
- World Water Week: Healthy ecosystems essential to human health: from coronavirus to malnutrition Online session Wednesday 24 August 17:00-18:20
- Mangrove Action Project Collaborates to Restore and Preserve Mangrove Ecosystems
Pollution
- Zakaria Ouedraogo of Burkina Faso Produces Film “Nzoue Fiyen: Water Not Drinkable”
- "Water and Sanitation-Related Diseases and the Changing Environment: Challenges, Interventions, and Preventive Measures" Volume 2 Is Now Available
Population
- "Water and Sanitation-Related Diseases and the Changing Environment: Challenges, Interventions, and Preventive Measures" Volume 2 Is Now Available
- "Water and Sanitation-Related Diseases and the Changing Environment: Challenges, Interventions, and Preventive Measures" Volume 2 Is Now Available
Public Health
- Honouring the visionary behind India’s sanitation revolution
- Honouring the visionary behind India’s sanitation revolution
Rivers
- World Water Week: Healthy ecosystems essential to human health: from coronavirus to malnutrition Online session Wednesday 24 August 17:00-18:20
- Mangrove Action Project Collaborates to Restore and Preserve Mangrove Ecosystems
Sanitation
- Honouring the visionary behind India’s sanitation revolution
- Honouring the visionary behind India’s sanitation revolution
Toxic Chemicals
- "Water and Sanitation-Related Diseases and the Changing Environment: Challenges, Interventions, and Preventive Measures" Volume 2 Is Now Available
- Actions to Prevent Polluted Drinking Water in the United States
Transportation
- "Water and Sanitation-Related Diseases and the Changing Environment: Challenges, Interventions, and Preventive Measures" Volume 2 Is Now Available
- Urbanization Provides Opportunities for Transition to a Green Economy, Says New Report
Waste Management
- Honouring the visionary behind India’s sanitation revolution
- Honouring the visionary behind India’s sanitation revolution
Water
- Honouring the visionary behind India’s sanitation revolution
- Honouring the visionary behind India’s sanitation revolution
Water and Sanitation
- Honouring the visionary behind India’s sanitation revolution
- Honouring the visionary behind India’s sanitation revolution

